Range of electrons in water and aluminium Fig 4.2 (1)
Published: Sep 29, 2021
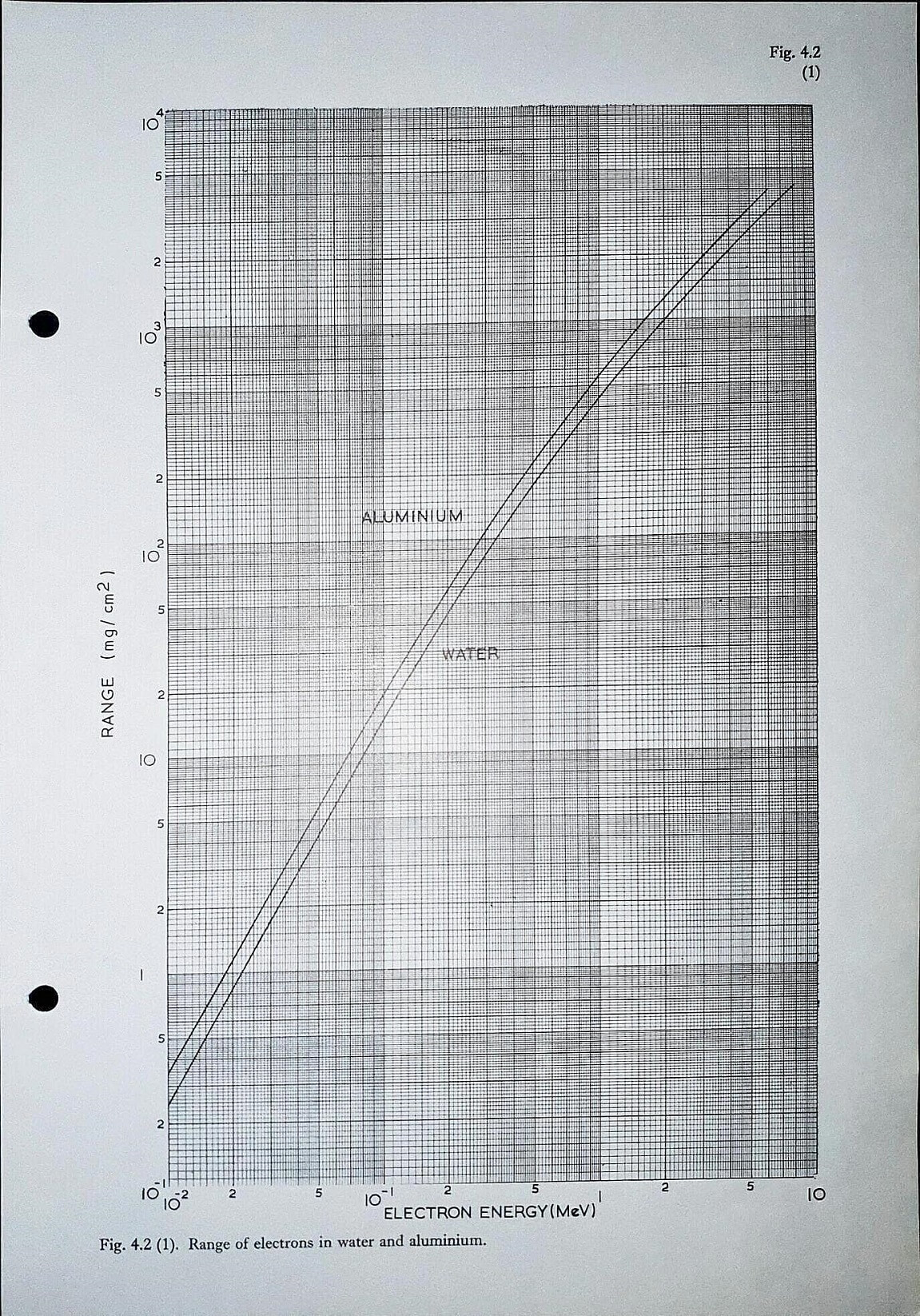
Discussion
Presented for academic interest more than anything. Note that the range is given in the units of mg/cm2. This is the mass of material per unit square area that is presented to the electrons. In order to turn the range into a linear value you need to divide by the density of the material.
For example, take a 1 MeV electron and examine its range through aluminium. We see from the above curve that the range is 450 mg/cm2. The density of aluminium is 2700 mg/cm3. Therefore, the linear range of the electron in this example will be about 1.67 mm. This is similar to that reported in other literature.
