A banana smoothie or a glass of tritiated wastewater from Fukushima Daiichi nuclear plant?
Published: Aug 23, 2023
We wrote this article for a bit of fun, if it is helpful or educational then all the better for it. This article is influenced by news that the Fukushima Daiichi nuclear plant will be releasing treated tritium aqueous waste into the sea under a controlled and authorised process. The aqueous waste water will have up to 1500 Bq of radioactive tritium per litre of liquid (this is further diluted once released into the sea). The becquerel (Bq) is the unit of radioactivity.
First we will provide a simplified summary - more like a statement of fact (based on the data we have). If the reader is interested and wants to dive deeper into this they can follow the derivation below the summary. An important outcome to take away from this article (media please note!), is that considering or comparing radioactivity (Bq) alone is not that helpful (it actually tells you very little). You should always consider the ionising radiation dose delivered if attempting to making comparisons to explain risk. This includes comparisons made between eating bananas and drinking tritiated waste water! Read on.
Summary
In the picture which follows below, on the left is an Ionactive banana smoothie. It is in a 1L glass and contains four pureed bananas with a little milk and oats to make up to 1L (1000 ml). Don’t worry about the size / mass of the bananas, it really does not matter considering the aim of this article (approximations will be fine).
On the right shows 1L of aqueous tritiated water from Fukushima Daiichi. We will assume that this has 1500 Bq of radioactive tritium (the agreed maximum release concentration).
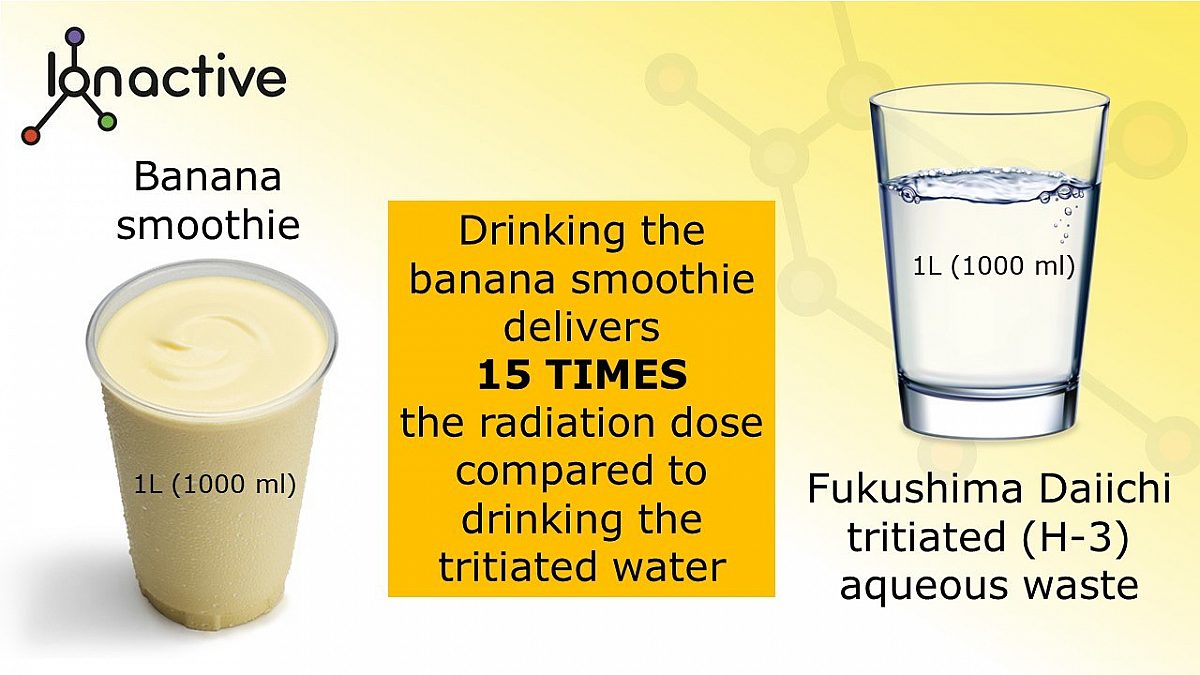
A banana smoothie vs tritiated wastewater from Fukushima Daiichi
Get this (!)
Drinking the banana smoothie will deliver a radiation dose (internal exposure) which is 15 TIMES HIGHER than drinking the 1L of aqueous tritiated water. Looking at this another way, you would need to drink 1L of aqueous tritiated water (right out of the discharge point) for 15 consecutive days, to achieve the same radiation dose delivered by just ONE of our lovely Ionactive banana smoothies. Or looking yet another way, and assuming a smoothie is not your thing, then eating less than 1/3 of a banana delivers a committed effective dose which is about the same radiation dose received from drinking the 1L of the tritiated wastewater.
This summary is not political. It does not intend to make a case specifically that exposures from tritium are as low as reasonably achievable (ALARA). We will let you decide that, partly based on the information in this article. If you want to know more than please read on.
The detail
Prelim
This is for readers who want some detail to back up the summary statement above. It is important to note that whilst we think the banana smoothie is healthy enough, consuming 1L per day every day is likely to be more than the average stomach and digestive system can stand (you have been warned!). Also, in the summary statement and this analysis, we are assuming that the Fukushima Daiichi tritiated water contains nothing but tritium (H-3) and water. We do not consider the chemical (non-radioactive) nature of the effluent.
Our site is full of useful resource and so we will not spend too much time defining terms – we have a radiation protection glossary for that.
The banana smoothie
Let us start with the banana smoothie. The banana is the key ingredients here – it does not actually matter if you eat them whole, cut up frozen or in a smoothie. A smoothie was chosen as it is somewhat liquid like, and therefore a comparator to other liquids (such as tritiated water). We chose four bananas based on looking up some example recipes to make up 1L (1000ml) of smoothie – in making the point in the summary statement, it really does not matter if you use 3 or 5 bananas, the same point is made.
Bananas contain the element potassium (symbol K), apparently essential for health and well-being. Out of all the naturally occurring potassium in the world, a small proportion will always be in the form of K-40 (potassium-40). K-40 is a naturally occurring radioactive substance and found in all foods (so please do not give up eating bananas!). K-40 has a half life of 1,300,000,000 years, so it has been around a long time and will continue to be so. Since K-40 is radioactive it will undergo decay (becoming Ca-40, 89% of the time via beta emission, and Ar-40 11% of the time via gamma emission). In other words we can state that K-40 is a beta / gamma emitter and the end result of the decay are stable isotopes. The key thing to note here is that beta / gamma radiation is released and this is what creates the radiation dose to the body when consuming the smoothie.
An average banana contains around 15 Bq of K-40 (where the Bq is an expression of how radioactive something is). Therefore, since the 1L smoothie contains four (average) bananas it follows that the K-40 radioactivity will be around 60 Bq.
[Note: if you are following this, you might already be jumping up and down shouting ‘ ..But …. But … the 1L of tritiated aqueous waste from Fukushima Daiichi to be released contains 1500 Bq/L of radioactivity – you lie Mr Ramsay …!! ‘. Well you are right – in so far as the ‘radioactivity’ of each example is concerned, but it is not about radioactivity, it is about DOSE (of radiation). So read on, there is no lie here].
The important thing to note with radioactivity (Bq) is that the type of radioactive material matters when considering radiation exposure. For example, the types radiation emitted and the energy of emission is important. So K-40 releases a gamma ray at an energy of 1.46 MeV (1,460,000 eV) for 11% of decays and a beta particle at an energy of 1.31 MeV (1,310,000 eV) for 89% of decays. In addition to this, the half-life (time taken for the activity to decay by half) and the chemical nature (non-radioactive quality) will influence how the dose is delivered (and by what mode – such as ingestion or inhalation). Inhalation of bananas is not recommended. Thankfully the ICRP (and others) have done the hard work and we can use various tools and tricks to come up with the ‘dose delivered’.
For this article we can use the data found in ICRP 109 (there is later data available but it makes negligible different, so sticking with what is easily found online and free etc). The key data is this:
- 6.2 x 10-9 Sv.Bq-1 . This is known as committed effective dose per unit intake. Let’s decode it.
- 6.2 x 10-9 can be written as 0.0000000062 (for the moment)
The Sv is the unit of effective dose, and for the purpose of this article means the dose received by the whole body. 1 Sv is a large dose of radiation, hence the small number appearing above.
Bq is the unit of activity as noted earlier. Committed means ‘it will be delivered’ – it is a bit more complicated than this, but essentially means that the dose delivered is calculated totally in the year of intake, but the dose might actually be delivered over a number of years (this all depends on the various factors noted earlier above). So we can read the expression above as:
- 0.0000000062 Sv per Bq of K-40 intake.
Most would say that 0.0000000062 is difficult number to read (which is why scientific notation is usually use).
What we can do is convert this to micro Sv noting that 1Sv = 1000 mSv – 1000,000 micro Sv. So if you grab your calculator, you will see we can now write that expression as:
- 0.0062 micro Sv per Bq of K-40 intake (much easier to read).
If you multiply that by the 60 Bq of K-40 in the Ionactive luscious banana smoothie you will see that drinking that 1L will yield a radiation does of 0.372 micro Sv (let’s round that upwards to 0.4 micro Sv).
So please keep that figure somewhere to hand. Things are about to get interesting (if you are not already dazzled already).
The 1L of tritiated wastewater from Fukushima Daiichi (up to 1500 Bq/L)
We now move on to look at the 1L of aqueous liquid containing up to 1500 Bq of tritium (H-3). At first glance it might look like the glass of water is more of a problem than the smoothie (which only has 60 Bq of radioactivity). Let’s look at tritium data in a little more detail.
Tritium (H-3) has a half-life of 12.3 years, so a much shorter half-life than K-40. Because it is so much shorter we can demonstrate half life in more meaningful detail (as compared to K-40). Imagine you sealed shut the 1L of tritiated water today, where 1500 Bq (per litre) has been measured. After 12.5 years there would only be 750 Bq remaining, and in a further 12.3 year there would only be 375 Bq remaining (and so on). Tritium decays to Helium-3 (He-3) which is non-radioactive.
Tritium (H-3) is a weak beta emitter (there are no gamma rays during the decay, unlike K-40 which is a beta / gamma emitter). Betas are emitted for 100% of decays at a maximum energy of 19 keV (19,000 eV). [Compare this to K-40 where the maximum beta energy is 1,310,000 eV – and recall again that K-40 is naturally occurring in the environment around you].
Using similar analysis for K-40, we can use the same ICRP publication to look for a committed effective dose per unit intake for H-3. This value is found to be as follows:
- 1.8 x 10-11 Sv.Bq-1. We can decode this as before and write in micro Sv which gives as the following.
- 0.000018 micro Sv per Bq of H-3 intake (much easier to read).
[Note: Compare this to K-40 where we came up with a value of 0.0062 micro Sv per Bq of K-40 intake].
Look carefully at the two values and you will see that the dose delivered per Bq of H-3 is FAR LESS then the dose delivered per Bq of K-40. However, we know we have more Bq of H-3 as stated earlier – so let’s finish this calculation off.
We have 1500 Bq x 0.000018 micro Sv per Bq (H-3) and this yields: 0.027 micro Sv.
Now let’s look at the comparison again between drinking our wonderful healthy Ionactive banana smoothie, and drinking the 1L of tritiated water from the Fukushima Daiichi nuclear plant. The results are in.
- Ionactive smoothie (K-40): 0.4 micro Sv (for 1L, 1000 ml)
- Fukushima Daiichi (H-3): 0.027 micro Sv (for 1L, 1000 ml)
WOW 😊
Look at the ratio of the two and we have 0.4 / 0.027 = 14.8 (15 rounded). And this is where we can back up the summary statement that drinking 1L of the Ionactive banana smoothie yields a committed effective dose which is 15 TIMES HIGHER than the dose received by drinking 1L of tritiated water from the nuclear plant.
In the UK the average person will receive about 3000 micro Sv/year from background radiation (radon, cosmic, food stuffs etc). So the doses for both the smoothie and the tritiated water are negligible.
Further considerations
Remember that the 1L of tritiated aqueous discharge will be diluted by the sea. The analysis above has assumed we are at the outlet of the aqueous discharge and filling up our 1L glass before any mixing with sea water.
Also note that environmental (naturally derived) levels of tritium exist in the ocean anyway. So millions of litres of tritiated water discharged still poses a negligible risk.
Let's finish by getting ridiculous (or perhaps not!). Consider the following picture.
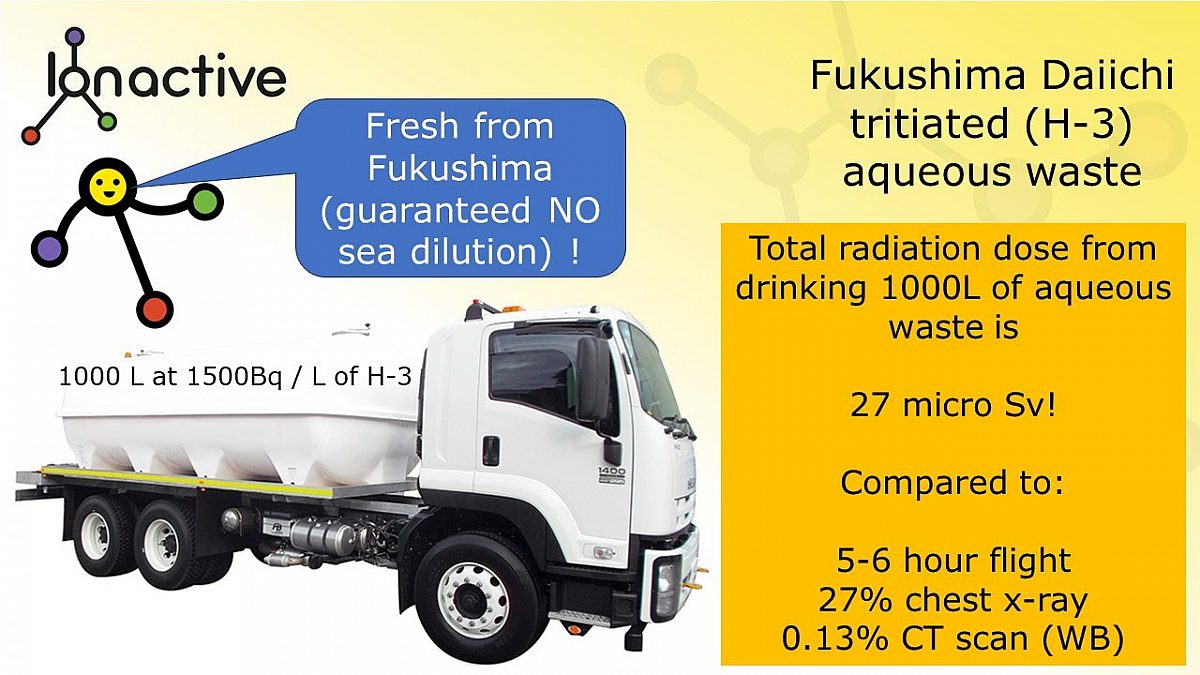
Tritiated (H-3) wastewater from Fukushima Daiichi - 1000 L in a tanker for drinking
Imagine having a tanker turn up at your house with 1000L of tritiated Fukushima Daiichi water (at 1500 Bq / L). This is all you have to drink. If over time you drink the 1000L of water it will deliver a total committed effective dose of about 27 micro Sv – that is about the same effective dose received during a 5-6 hour flight at 38,000 feet (external dose from cosmic radiation).
Effective dose (Sv) is useful as we can compare radiation dose (risk) directly from intakes of radioactive material and external irradiation (in the case of cosmic radiation).
Dose equivalent banana is not perfect
The dose equivalent banana (BED) is far from perfect. To read up on this, go to our article: BED. Basically the body regulates potassium (K), and therefore K-40. So you cannot "overdose" on a banana (or any other food stuff containing K-40) from a radiation exposure perspective. As you take in K, the body will excrete K. So the above comparison between H-3 and K-40 is best used as a side by side single exposure comparison. The risk findings at a moment in time are correct, just do not over do the banana!
